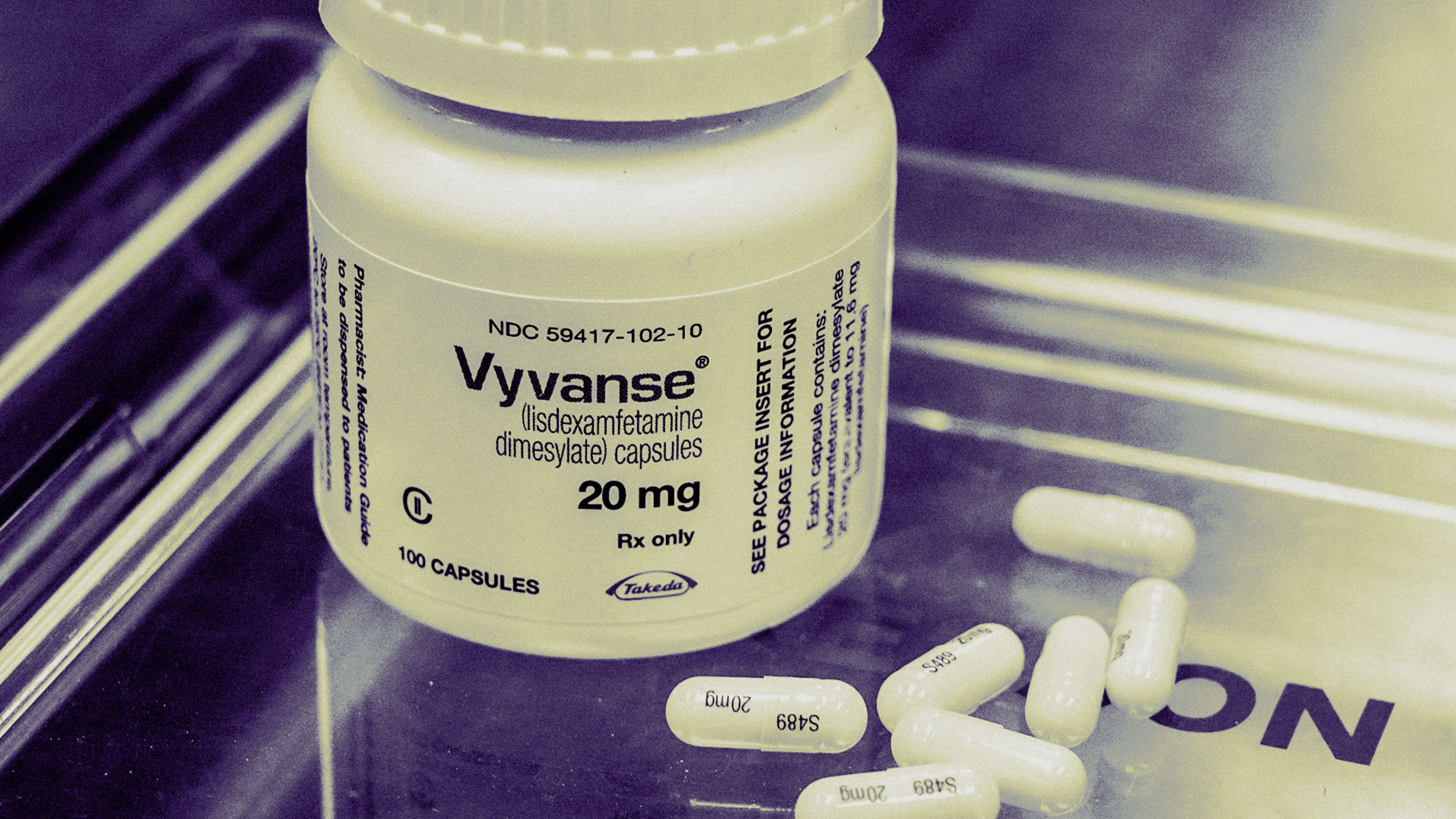
Nov 1, 2024 · soon after vyvanse’s patent expired in 2023, applications for generic formulations of lisdexamfetamine dimesylate started flowing into the fda. There are now 18 approved. Us drug authorities are allowing 24% more production of vyvanse, an adhd drug from takeda pharmaceutical co. And generic rivals, to address treatment shortages that have dragged on. Sep 5, 2024 · the u. s.
Nov 11, 2024 · from 1 december 2024, lisdexamfetamine (vyvanse) will be listed in section b and part ii of section h of the pharmaceutical schedule as follows: A confidential rebate will. Sep 6, 2024 · vyvanse shortage a similarly complicated situation is seen with the current shortfall of vyvanse and its generics. The dea raised the quota after prodding from the food and drug. 6 days ago · sun pharma has lisdexamfetamine capsules on shortage due to an issue with the active ingredient. Takeda has vyvanse available. Teva has lisdexamfetamine capsules. Sep 4, 2024 · the u. s. Drug enforcement administration has increased the production limit for takeda pharmaceutical's adhd drug vyvanse and its generic versions by about 24% to.
The Leak That Changed Everything: Cruella De Vil's Private Life!
Jen Bretty Leak: The Key Players Revealed
Unseen Jenna Lynn Meowri: What Happened After The Leak